Dr. S Adimurthy's Group


Publications
2024
108. Sustainable N-Formylation of Anilines: Harnessing Aleuritic Acid as a Renewable Formyl Source Hetvi A Vadariya, Gaurav Badhani, B. Mohamed Farves, Krupa N. Boda, S. Adimurthy* SYNLETT, 2024, xxx–xxx. DOI: 10.1055/a-2388-9578
107. Lewis–acid mediated isothiocyanation and chlorination of quinoxalin-2(1H)-ones under visible light conditions Gaurav Badhani, Shubham, B. Valvi Mangesh, and S. Adimurthy* J. Org. Chem. 2024, 89, 10760–10772. https://doi.org/10.1021/acs.joc.4c00995
106. Book publication: Eco-Friendly Bromination and Oxybromination of Diverse Organic Molecules By S. Adimurthy, Chitrakar Ravi and Rajendra D. Patil
First published 2024 (12/02/2024). Pages 266. Cambridge Scholars Publication, UK.
ISBN (10): 1-5275-6392-8; ISBN (13): 978-1-5275-6392-6
https://www.cambridgescholars.com/product/978-1-5275-6392-6
105. Arylation of quinoxalinones at room temperature under metal and base free-conditions Gaurav Badhani and S. Adimurthy* Monatshefte für Chemie - Chemical Monthly 2024, 155, 613– 619. DOI : 10.1007/s00706-024-03188-2
2023
104. Ionic Liquid–Catalysed Regioselective Oxygenation of Quinoxalin-2(1H)-ones Under Visible-Light Conditions Gaurav Badhani, Valvi Mangesh Biramya and S. Adimurthy* New J. Chem., 2023, 47, 21596 – 21599. DOI: 10.1039/D3NJ04496C
103. Book Chapter on “L-Proline catalyzed transamidation of thioamides with amines” S. Nageswara Rao, D. Chandra Mohan and S. Adimurthy* 2023, Vol 8, chapter 9, pages. 123-133.DOI: 10.9734/bpi/ctcb/v8/4940E ; Publisher: B.P. International, Editor: Dr. Farzaneh Mohamadpour; Book name: Current Topics on Chemistry and Biochemistry Print ISBN: 978-81-19039-31-9, eBook ISBN: 978-81-19039-30-2
102. Water-Mediated C–H Cyanation of Quinoxalin-2(1H)-ones and Quinoxalines Under Visible-Light Conditions Gaurav Badhani and S. Adimurthy* ChemistrySelect 2023, 8, e202302159 https://doi.org/10.1002/slct.202302159
2022
102. Recovery of lac resin from the aqueous effluent of shellac industry Gaurav Badhani, Shruti Yadav, Elen Reji, S. Adimurthy* Sustain. Chem. 2023, 4(1), 1-7; https://doi.org/10.3390/suschem4010001 (online 21/12/2022). (This article published under the Special Issue Alternative Solvents for Green Chemistry)
101. Palladium–Catalyzed Regioselective C–H Heteroarylation of Pyridotriazoles Deepa Rawat, Semwal Rashmi and S. Adimurthy* ChemistrySelect, 2022, 7, (33), xx, (available online 01/9/2022) https://doi.org/10.1002/slct.202202456. DOI: 10.1002/slct.202202456 [I F = 2.307].
100. Pd-Catalyzed annulation of imidazo[1,2-a]pyridines with coumarins and indoles: Synthesis of benzofuran and indole fused heterocycles Rashmi Semwal, Gaurav Badhani and S. Adimurthy* Chem. Comm. 2022, 58, 1585–1588. (online 24/12/2021) DOI: 10.1039/D1CC06803B [I.F. = 6.222].
99. Transannulation of pyridotriazoles with naphthoquinones and indoles: synthesis of benzo[f]pyrido[1,2-a]indoles and indolizino[3,2-b]indoles Deepa Rawat and S. Adimurthy* Adv. Synth. Catal. 2022, 364, 71–76. Online 14th Oct, 2021 (DOI 10.1002/adsc.202100965) [I.F. = 5.837].
2021
98. Ionic-Liquid-Catalyzed Synthesis of Imines, Benzimidazoles, Benzothiazoles, Quinoxalines and Quinolines through C-N, C-S, and C-C Bond Formation Gaurav Badhani, Abhisek Joshi, S. Adimurthy* 2021, Eur. J. Org. Chem. 2021, 6705–6716. DOI: 10.1002/ejoc.202101135 https://doi.org/10.1002/ejoc.202101135 [I.F. = 3.029].
97. BF3·Et2O Catalyzed Transannulation of Pyridotriazoles with Isothiocyanates: Synthesis of Thiazolo[3,4-a]pyridin-3-imines Rahul Kumar, V. Ginoya, Rashmi Semwal and S. Adimurthy* New J. Chem. 2021, 45, 20547–20550. DOI:10.1039/d1nj04033b (online 18/10/2021) [I.F. = 3.591].
96. Ru-Catalyzed Selective C-H functionalization of pyridotriazoles with acrylates
Abhisek Joshi, Semwal Rashmi, S. Adimurthy SynOpen, 2021, 5, 294–300. (on line 5/10/2021) 10.1055/a-1661-5655 (thieme-connect.com)
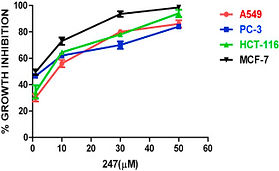
95. Design, Synthesis and Anticancer activity of Sulfenylated Imidazo-Fused Heterocycles C. Ravi, Deepa Rawat, Sistla Ramakrishna,* V. Lakshma Nayak and S. Adimurthy* Bioorg. Med. Chem. Lett. 2021, 49, 128307. https://doi.org/10.1016/j.bmcl.2021.128307 [I.F. = 2.823].
94. Hypervalent Iodine Mediated Synthesis of Imidazo[1,2-a] pyridine Ethers: Consecutive Methylene Linkage and Insertion of Ethylene Glycol R. Kumar, D. Rawat, Semwal Rashmi, Gourav Bhadani and S. Adimurthy* New J. Chem. 2021, 45, 7491–7495. https://doi.org/10.1039/D1NJ00657F DOI: 10.1039/D1NJ00657F [I.F. = 3.288].
93. Synthesis of Thiazolidinimines/Thiazinan-2-imines via Three Component Coupling of Amines, vic-Dihalides and Isothiocyanates Under Metal-free Conditions R. Kumar, A. Joshi, D. Rawat and S. Adimurthy* Synth. Commun. 2021, 51, 1340–1352. DOI: 10.1080/00397911.2021.1880594 [I F = 1.796]
2020
92. Annulation of Imidazo[1,2-a]pyridines Under Metal-free Conditions Rashmi Semwal, Abhisek Joshi, Rahul Kumar and S. Adimurthy* New J. Chem. 2020, 44, 20530–20534. (https://doi.org/10.1039/D0NJ04521G) [I.F. = 3.288].
91. Visible−Light Induced Phosphonation of Quinoxalines and Quinoxalin-2(1H)-ones Under Aerobic Metal-free Conditions Deepa Rawat, Rahul Kumar and S. Adimurthy, Green Chem., 2020, 22, 6170–6175. https://doi.org/10.1039/D0GC02168G
97. Polyethylene Glycol (PEG-400): As Methylene Spacer and Green Solvent for the Synthesis of Hetero Diarylmethanes Under Metal-Free Conditions Rahul Kumar, Deepa Rawat and S. Adimurthy* Eur. J. Org. Chem. 2020, 3499−3507. (Available online DOI: 10.1002/ejoc.202000467) [I.F. = 3.029].
90. An integrated effluent free process for the production of 5-hydroxymethyl furfural (HMF), levulinic acid (LA) and KNS-ML from aqueous seaweed extract Faisal Kholiya, Meena R. Rathod, D. R Gangapur, S. Adimurthy,* Ramavatar Meena* Carbohydrate Research, 2020, 490, 107953. (https://doi.org/10.1016/j.carres.2020.107953) [I F =1.873]
89. Polyaniline@porous polypropylene for efficient separation of acid by diffusion dialysis Pradeep K Prajapati, N. Naresh K Reddy, Raghavendra Nimiwal, Puyam Singh, S. Adimurthy and R. Nagarale Separation and Purification Technology, 2020, 233, 115989. https://doi.org/10.1016/j.seppur.2019.115989 (online 27/8/2019) [I.F. 5.107].
2019
88. Pd-Catalyzed ortho Selective C-H Acyloxylation and Hydroxylation of Pyridotriazoles D. Rawat, R. Kumar, and S. Adimurthy Eur. J. Org. Chem. 2019, 7874−7879.
87. Copper-Catalysed Multi-Component Reactions (MCR’s) for Disulfenylation of Imidazo[1,2-a]pyridines using Elemental Sulfur and Arylhalides and Intramolecular Cyclisation of Haloimidazo[1,2-a]pyridines S. Rashmi, C. Ravi, S. Soumya S. Adimurthy* J. Org. Chem. 2019, 84, 14151−14160.
86. Catalyst-free Azo-arylation of Arenes/Heteroarenes at Room Temperature Rahul Kumar, C. Ravi, A. Joshi, R. Semwal and S. Adimurthy* ChemistrySelect 2019, 4, 5740–5744.
85. Indium catalyzed denitrogenative transannulation of pyridotriazoles: Synthesis of pyrido[1,2-a]indoles Deepa Rawat, C. Ravi, A. Joshi, E. Suresh*, K. Jana, B. Ganguly* and S. Adimurthy* Org. Lett. 2019, 21, 2043–2047.
84. Ionic Liquid Catalysed Aerobic Oxidative Amidation and Thioamidation of Benzylic Amines under Neat Conditions A. Joshi, Rahul Kumar, Rashmi Semwal, Deepa Rawat, and S. Adimurthy* Green Chem. 2019, 21, 962–967.
83. Sodium salts (NaI/NaBr/NaCl) for the halogenation of imidazo-fused heterocycles S. Rashmi, C. Ravi, Rahul Kumar, R. Meena and S. Adimurthy* J. Org. Chem. 2019, 84, 792−805. 10.1021/acs.joc.8b02637
2018
82. Visible-Light Induced C (sp3)–H Functionalization of Tosylhydrazones: Synthesis of Polysubstituted Pyrroles under Metal-free Conditions N. Naresh K. Reddy, Deepa Rawat, S. Adimurthy* J. Org. Chem. 2018, 83, 9412–942.
81. Transition metal-free hydration of nitrilesto amides mediated by NaOH
N. N. K. Reddy, S. Nageswara Rao, R. D. Patil and S. Adimurthy*
Adv. Mater. Sci., Open Access Text. 2018, 3, 1-7. doi:10.15761/AMS.1000137
80. Iodine-catalyzed One-pot Decarboxylative Sulfenylation of Electron rich Arenes and Indoles
C. Ravi, Rashmi Semwal, Rahul Kumar, N. N. K. Reddy and S. Adimurthy*
ChemistrySelect 2018, 3, (in press, DOI: 10.1002/slct.201801119).

79.Base-Promoted Transition metal-free Arylation of Imidazo fused Heterocycles with Diaryliodonium salts
Rahul Kumar, Chitrakar Ravi, Deepa Rawat and Subbarayappa Adimurthy*
Eur. J. Org. Chem., DOI: http://dx.doi.org/10.1002/ejoc.201800286
2017
78.Nitric Acid Assisted In Situ Generation of BrOH: A Selective Catalyst for Oxidation of Benzylic Alcohols
P. Venkatanarayana and S. Adimurthy*
Journal Organic and Inorganic Chemistry, 2017, 3, (2), 1-5.
DOI: 10.21767/2472-1123.100024
Supravat Samanta, Chitrakar Ravi, Sadu Nageswara Rao, Abhisek Joshi and Subbarayappa Adimurthy *
Org. Biomol. Chem., 2017,15, 9590-9594.
75.Catalyst-Free Synthesis of 2,4-Disubstituted‑1H‑imidazoles through[3 + 2] Cyclization of Vinyl Azides with Amidines
N. Naresh Kumar Reddy, Sadu Nageswara Rao, Chitrakar Ravi, and Subbarayappa Adimurthy*
ACS Omega 2017, 2, 5235−5241

74. Oxidative Amidation of Methylarenes and Heteroamines under Metal-Free Conditions
Venkatanarayana Pappula, Chitrakar Ravi, Supravat Samanta, and Subbarayappa Adimurthy*
ChemistrySelect 2017, 2, 5887 – 5890.
73. C‐3 Sulfenylation of N‐heteroarenes in water under catalyst‐free conditions
Chitrakar Ravi, Abhisek Joshi, and Subbarayappa Adimurthy*
Eur. J. Org. Chem. 2017, 3646–3651

72. Synthesis of imidazo[1,2-a]pyridines: C-H functionalization in the direction of C-S bond formation
Chitrakar Ravi, and Subbarayappa Adimurthy*
The Chemical Record, DOI:10.1002/tcr.201600146
71. Synthesis of Functionalized Pyrazolo[1,5-a]pyridines: [3+2] Cycloaddition of N-Aminopyridines and α,β-Unsaturated Carbonyl Compounds/Alkenes at Room Temperature
Chitrakar Ravi, Supravat Samanta, D Chandra Mohan, N. Naresh Kumar Reddy, Subbarayappa Adimurthy*
70. Visible-light- induced aerobic dioxygenation of styrenes under metal- and additive-free ambient conditions
Supravat Samanta, Chitrakar Ravi, Abhisek Joshi, Venkatanarayana Pappula, Subbarayappa Adimurthy*
Tetrahedron Letters 2017, DOI: org/10.1016/j.tetlet.2016.12.073
69. Design, synthesis and cytotoxicity studies of novel pyrazolo[1, 5-a]pyridine derivatives
Chitrakar Ravi, Arem Qayum, Darapaneni Chandra Mohan, Shashank K. Singh, Subbarayappa Adimurthy*
Eur.J. Med Chem. 2017, 126, 277–285. DOI: org/10.1016/j.ejmech.2016.11.037
2016
68. Copper-Catalyzed Three-Component System for Arylsulfenylation of
Imidazopyridines with Elemental Sulfur
Chitrakar Ravi, N. Naresh Kumar Reddy, Venkatanarayana Pappula, Supravat Samanta,
and Subbarayappa Adimurthy*
J. Org. Chem. 2016, 81, 9964−9972
DOI: 10.1021/acs.joc.6b01715
67. Lewis Acid-Catalyzed Denitrogenative Transannulation of Pyridotriazoles with Nitriles: Synthesis of Imidazopyridines
Abhisek Joshi, Darapaneni Chandra Mohan, and Subbarayappa Adimurthy*
J. Org. Chem. 2016, 81, 9461−9469
DOI: 10.1021/acs.joc.6b01742
66. Green process development for the preparation of 2,6-dibromo-4-nitroaniline from 4-nitroaniline using bromide–bromate salts in an aqueous acidic medium
Venkatanarayana Pappula and Subbarayappa Adimurthy*
RSC Adv., 2016, 6, 90184–90187
DOI: 10.1039/c6ra13680j
65. Iodine-catalyzed [3+2] cyclization of 2-pyridylesters and chalcones: metal-free approach for the synthesis of substituted indolizines
N. Naresh Kumar Reddy, Ramachandra Reddy Donthiri, Chitrakar Ravi, Subbarayappa Adimurthy*
Tetrahedron Letters. 2016, 57, 3243–3246.
64. AIBN-promoted amidation of anilines with 1, 3-diketones via oxidative cleavage of C–C bond under aerobic conditions
Sadu Nageswara Rao, Darapaneni Chandra Mohan, Subbarayappa Adimurthy*
Tetrahedron. 2016, 72, 4889–4894.
63. Phenyliodonium Diacetate Mediated Oxidative Functionalization of Styrenes with Molecular Oxygen: Synthesis of α‑Oxygenated Ketones
Supravat Samanta, Ramachandra Reddy Donthiri, Chitrakar Ravi, and Subbarayappa Adimurthy*
J. Org. Chem. 2016, 81, 3457−3463
doi:10.1021/acs.joc.6b00266
62. Metal–free Synthesis of Indolizines through Oxidative C–C and C–N Bond Formations of C (sp3) –H Bonds
N. Naresh Kumar Reddy, Darapaneni Chandra Mohan, Subbarayappa Adimurthy *
Tetrahedron Letters
doi:10.1016/j.tetlet.2016.01.082g
61. Dual Role of p–Tosylchloride: Copper–Catalyzed Sulfenylation and Metal free Methylthiolation of Imidazo [1, 2–a] pyridines
Chitrakar Ravi, Darapaneni Chandra Mohan and Subbarayappa Adimurthy *
Org. Biomol. Chem.
DOI: 10.1039/c5ob02475g
60. Copper-Catalyzed Denitrogenative Transannulation Reaction of Pyridotriazoles: Synthesis of Imidazo[1,5‑a]pyridines with Amines and Amino Acids
Abhisek Joshi, Darapaneni Chandra Mohan and Subbarayappa Adimurthy *
Organic Letters
DOI: 10.1021/acs.orglett.5b035097
2015
59. H-β-zeolite catalyzed transamidation of carboxamides, phthalimide, formamides and thioamides with amines under neat conditions
Sadu Nageswara Rao, Darapaneni Chandra Mohan and Subbarayappa Adimurthy *
RSC Adv., 2015, 5, 95313-95317
58. Copper catalyzed C(sp3)−H functionalization of deoxybenzoins with vinyl azides: Synthesis of 2, 3, 5−triphenyl−1H−pyrroles
Ramachandra Reddy Donthiri, Supravat Samanta and Subbarayappa Adimurthy*
Org. Biomol. Chem., 2015,13, 10113-10116
57.Iodine Catalysed Intramolecular C(Sp3)−H Functionalization: Synthesis of 2, 5−disubstituted Oxazoles from N−Arylethylamides
Supravath Samantha, Ramachandra Reddy Donthiri, Milan Dinda and Subbarayappa Adimurthy
RSC Adv., 2015,5, 66718-66722
56. Synthesis of Indolizines through Oxidative Linkage of C–C and C–N Bonds from 2-Pyridylacetates
Darapaneni Chandra Mohan , Chitrakar Ravi , Venkatanarayana Pappula and Subbarayappa Adimurthy
J. Org. Chem., 2015, 80, 6846–6855, (Top most-accessed article in month of July 2015),
55. Substrate selective synthesis of pyrazolo[1,5-a]pyridines through [3 + 2] cycloaddition of N-aminopyridines and β-nitro styrenes
Chitrakar Ravi, Darapaneni Chandra Mohan, N. Naresh Kumar Reddy and Subbarayappa Adimurthy
RSC Adv., 2015, 5, 42961-42964, Link
54. Copper-catalyzed aerobic oxidative amination of C(sp3)–H bonds: synthesis of imidazo[1,5-a]pyridines
Darapaneni Chandra Mohan, Sadu Nageswara Rao, Chitrakar Ravi and Subbarayappa Adimurthy
Org. Biomol. Chem., 2015, 13, 5602-5607 (Top most-accessed article in month of April 2015)
53. Copper-mediated synthesis of pyrazolo[1,5-a]pyridines through oxidative linkage of C–C/N–N bonds
Darapaneni Chandra Mohan, Chitrakar Ravi, Sadu Nageswara Rao and Subbarayappa Adimurthy
Org. Biomol. Chem., 2015, 13, 3556. (Top most-accessed article in month of February 2015)
2014
I
52. Copper-Catalyzed C–H Functionalization of Pyridines and Isoquinolines with Vinyl Azides: Synthesis of Imidazo Heterocycles
Ramachandra Reddy Donthiri, Venkatanarayana Pappula, N. Naresh Kumar Reddy, Dipayan Bairagi, and Subbarayappa Adimurthy, J. Org. Chem., 2014, 79 ,11277–11284.
51.Transition metal-free oxidative esterification of benzylic alcohols in aqueous medium
Supravat Samanta, Venkatanarayana Pappula, Milan Dinda and Subbarayappa Adimurthy,
Org. Biomol. Chem., 2014,12, 9453-9456,
50. Chitosan: an efficient recyclable catalyst for transamidation of carboxamides with amines under neat conditions
Sadu Nageswara Rao, Darapaneni Chandra Mohan and Subbarayappa Adimurthy
Green Chem., 2014,16, 4122-4126, (Top most-accessed article in month of August 2014)
49. H-β-zeolite catalyzed synthesis of β-bromostyrenes from styrene bromohydrins
Venkatanarayana Pappula, Ramachandra Reddy Donthiri, Chandra Mohan Darapaneni, Adimurthy Subbarayappa,
Tetrahedron Letters 2014, 55 1793.
48. N-Chlorosuccinimide-Promoted Regioselective Sulfenylation of Imidazoheterocycles at Room Temperature
Chitrakar Ravi, Darapaneni Chandra Mohan, and Subbarayappa Adimurthy
Org. Lett., 2014, 16, 2978. (Top most-accessed article in month of July 2014)
I
47.Copper(I) Iodide Catalyzed Aerobic Oxidative C-N and C-S bond formations through C-H Activation: Synthesis of Functionalized Imidazo [1,2-a]pyridines
Darapaneni Chandra Mohan, Sadu Nageswara Rao, Chitrakar Ravi and Subbarayappa Adimurthy
Asian J. Org.Chem. 2014, 3, 609. (Top most-accessed article in month of June 2014),
2013
46. Bromide-bromate Couple of Varying Ratios for Bromination, Vicinal Functionalisation and Oxidation in a Clean Manner
Subbarayappa Adimurthy, Brindaban C. Ranu, Gadde Ramachandraiah, Bishwajit Ganguly and Pushpito K. Ghosh
Current Organic Synthesis, 2013, 10, 864-884
45. Water mediated deprotective intramolecular hydroamination of N-propargylaminopyridines: synthesis of imidazo[1,2-a]pyridines
Darapaneni Chandra Mohan, Niraj B. Sarang, Subbarayappa Adimurthy
Tetrahedron letters 2013, 54, 6077,
44. Copper(I) Iodide-Catalysed Aerobic Oxidative Synthesis of Imidazo[1,2-a]pyridines from 2-Aminopyridines and Methyl Ketones
Darapaneni Chandra Mohan, Ramachandra Reddy Donthiri, Sadu Nageswara Rao and Subbarayappa Adimurthy
Advanced Synthesis & Catalysis 2013, 355, 2217. (Top most-accessed article in month of September 2013)
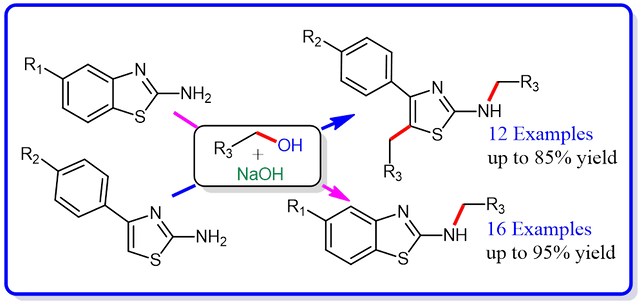
43.Sodium Hydroxide Catalyzed N-Alkylation of (Hetero) Aromatic Primary Amines and N1,C5-Dialkylation of 4-Phenyl-2-aminothiazoles with Benzyl Alcohols
Ramachandra Reddy Donthiri, Venkatanarayana Pappula, Darapaneni Chandra Mohan, Hiren H. Gaywala, and Subbarayappa Adimurthy
J. Org. Chem., 2013, 78 (13), pp 6775–6781.
42. L-Proline: An Efficient Catalyst for Transamidation of Carboxamides with Amines
Sadu Nageswara Rao, Darapaneni Chandra Mohan, and Subbarayappa Adimurthy
Org. Lett., 2013, 15, 1496. (Top most-accessed article in month of April 2013)
41.Catalytic Methods for Synthesis Imine
Rajendra D. Patil and Subbarayappa Adimurthy
Asian J. Org. Chem. 2013, 2, 726–744, (Top most-accessed article in year of 2013)
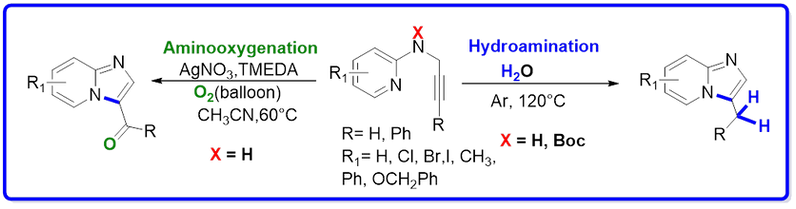
40. Synthesis of Imidazo[1,2-a]pyridines: “Water-Mediated” Hydroamination and Silver-Catalyzed Aminooxygenation
Darapaneni Chandra Mohan , Sadu Nageswara Rao, and Subbarayappa Adimurthy
J. Org. Chem. 2013, 78, 1266. (Top most-accessed article in month of February 2013), Link
39. KHSO4-catalyzed green synthesis of 1,1'-oxybis(2-bromoethane-1,1-diyl) dibenzenes and 1-(1-(benzyloxy)-2-bromoethyl)benzenes under solvent free conditions
G. Joshi, E. Suresh and S. Adimurthy
SyntheticCommun. 2013, 43, 587-599. DOI: 10.1080/00397911.2011.604816.
2012
38. Clean synthesis of crystalline p-nitrobenzyl bromide from p-nitrotoluene with zero organic discharge
M. Dinda, M. K. Agrawal, M. R. Gandhi, S. C. Upadhyay, S. Adimurthy, S. Chakraborty, and P. K. Ghosh
RSC- Advances 2012, 2, (16) 6645-6649. DOI: 10.1039/c2ra20940c.
37. NaOH—Catalyzed imines syntheses: aerobic oxidative coupling of alcohols and amines
D. Ramachandra Reddy, R.D. Patil and S. Adimurthy
Eur. J. Org. Chem. 2012, (24) 4457-4460. DOI: 10.1002/ejoc.201200716.
(The article has been cited under most accessed articles ofJuly2012)
36. H-β-Zeolite Catalysed Hydroarylation of Styrenes
D. Chandramohan, R.D. Patil and S. Adimurthy
Eur. J. Org. Chem. 2012, (8), 3520-3525. DOI: 10.1002/ejoc.201200283.
35. Copper(0)—Catalyzed Selective Aerobic Oxidation of Amines to Imines under Solvent-Free Conditions
R. D. Patil and S. Adimurthy
RSC Advances 2012, 2, 5119-5122. DOI:10.1039/C2RA20339A.
34. Oxidative Esterification of Benzaldehyde and Deactivated Aromatic Aldehydes with N-Bromosuccinimide-Pyridine
M. K. Agrawal, S. Adimurthy, P. K. Ghosh
Synthetic Commun. 2012, 42, 2931-2936, DOI:10.1080/00397911.2011.572219.
33. Green Bromine: In-situ Generated Catalyst for Selective Oxidation of Benzylic/Secondary Alcohols
G. Joshi, R.D. Patil S. Adimurthy
RSC Advances, 2012, 2, (6) 2235-2239. DOI:10.1039/C2RA20073B.
2011
32. Environment-Friendly Bromination of Aromatic Heterocycles using Bromide-Bromate Couple in Aqueous Medium
G. Joshi, S. Adimurthy
Industrial & Engineering Chemistry Research 2011, 50, 12271-12275. DOI: 10.1021/ie2004863.
31. Copper-Catalyzed Aerobic Oxidation of Amines to Imines Under Neat Conditions With Low Catalyst Loading
R. D. Patil and S. Adimurthy
Adv. Synth. Cat. 2011, 353, 1695-1700. DOI:10.1002/adsc.201100100. (The article has been cited under most accessed articles of 2011)
30. Direct and selective conversion of benzyl bromides to benzaldehydes with aqueous H2O2 without catalyst
R. D. Patil and S. Adimurthy*
Synth. Commun. 2011, 41, (18), 2712-2718. DOI:10.1080/00397911.2010.515347
29. Catalyst- and Solvent- Free Synthesis of Substituted Imidazo[1,2-a]pyridines
K. C. Chunavala, G. Joshi, E. Suresh and S. Adimurthy
SYNFACTS 2011, (5), 478.
28. Iodineand indium(iii)chloride catalyzed facile synthesis of 1,5- and 1,8-naphthyridines
K. C. Chunavala and S. Adimurthy
Synth. Commun. 2011, 41, 1843-1851. DOI:10.1080/00397911.2010.493261
27. FeSO4: A substitute to benzyloxypyridinium triflates for synthesis of benzyl alkyl ethers without base
Girdhar Joshi and S. Adimurthy Synth. Commun. 2011, 41, (5), 720-728. DOI:10.1080/00397911003642674
26. Thermal and Microwave Assisted Rapid Synthesis of Substituted Imidazo[1,2-a]pyridines Under Solvent and Catalyst free Conditions
K. C. Chunavala, G. Joshi, E. Suresh and S. Adimurthy
Synthesis 2011, (4) 635-641. Doi:10.1055/s-0030-1258405. (This work has been selected for Organic Chemistry Portal under Org. Chem. Abstracts http://www.organic-chemistry.org/abstracts/lit3/170.shtmTwo types of categories one is Synthesis of N-heterocycles (synthesis of azaindoles) http://www.organic-chemistry.org/synthesis/heterocycles/azaindoles.shtm and other one is Green Chemistry http://www.organic-chemistry.org/topics/green-chemistry.shtm)
2010
25. High atom efficient and environment-friendly preparation of herbicides bromoxynil and ioxynil
S. Adimurthy, G. Joshi and R. D. Patil
Indian J. Chem. 2010, 49B, 1678-1680.
24. Easy access to a-bromoketones and epoxides from vic-dibromides under aqueous conditions
R. D. Patil, S. Adimurthy, B.C. Ranu
Synth. Commun. 2010, 40, 3233-3239.
23. KHSO4-A highly efficient and reusable heterogeneous catalyst for hydroarylation of styrenes
R. D. Patil, G. Joshi and S. Adimurthy
Chemical Monthly 2010, 141, 1093-1099. DOI 10.1007/s00706-010-0369-2.
22. A green oxidation of methylarenes to benzoic acids with bromide/bromate couple in water R. D. Patil,S. Bhadra, S. Adimurthy and B. C. Ranu
Synthetic Commun. 2010, 40, 2922-2929.
21. HBr-H2O2: A Facile protocol for regioselective synthesis of bromohydrin, a-bromoketones and oxidation of benzylic/secondary alcohols to carbonyl compounds under mild aqueous conditions
R. D. Patil, G. Joshi, S. Adimurthy
Industrial & Engineering Chemistry Research 2010, 49, 8100-8105. DOI:10.1021/ie100492r
20. A Fast and Highly Efficient Method for the Synthesis of Tertiary Amines in Aqueous Medium
S. Adimurthy and G. Joshi
Indian J. Chem. 2010, 49B, 771-775.
19. Bromide tolerance in plants- A case study on halophytes of Indian coast
M. S. Reddy, M. P. Joshi, S. P. Dave, S. Adimurthy, V. R. K.S. Susarla, A. S. Mehta, P.V. Subbarao, M. P. Reddy and G. Ramachandraiah
Scholarly Research Exchange Ecology (SRX Ecology)2010, 1-6. Doi:10.3814/2010/650678
18. Making Full Use of the Oxidizing Equivalents in Bromate in the Selective Oxidation of Thiols, Sulfides and Benzylic/Secondary Alcohols into Disulfides, Sulphoxides and Aldehydes/Ketones G. Joshi, S. Bhadra, S. Ghosh, M. K. Agrawal, B. Ganguly, S. Adimurthy, P. K. Ghosh, B. C. Ranu
Industrial & Engineering Chemistry Research 2010, 49, 1236-1241.
2009
17. The Influence of Bases and Ligands on the Outcome of the Cu(I)-Catalyzed Oxidative Homocoupling of Terminal Alkynes to 1,4-Disubstituted 1,3-Diynes Using Oxygen as an Oxidant
S. Adimurthy, Chandi. C. Malakar and U. Beifuss
J. Org. Chem. 2009, 74, 5648-5651. DOI: 10.1021/jo900246z
16. An Efficient Method for the Synthesis of 2,3-Dihydro-1H-Isoindoles
S. Adimurthy and P. U. Patoliya
Indian J. Chem. 2009, 48B, 545-552.
15. Facile and One Pot Synthesis of a-Bromoketones from Olefins With an Alternative Brominating agent
R. D. Patil, G. Joshi, S. Adimurthy, B.C. Ranu.
Tetrahedron Lett. 2009, 50, 2529-2532. Doi:10.1016/j.tetlet.2009.03.047
14. Comparative study of the vicinal functionalization of olefins with 2:1 bromide-bromate and iodide-iodate reagents
M. K. Agrawal, S. Adimurthy, B. Ganguly, P. K. Ghosh
Tetrahedron 2009, 65, 2791-2797.
2003-2008
13. An Alternative Method for the Regio- and Stereoselective Bromination of Alkenes, Alkynes, Toluene Derivatives and Ketones Using a Bromide/Bromate Couple
S. Adimurthy, S. Ghosh, P. U. Patoliya, G. Ramachandraiah, Manoj Agrawal, M. R. Gandhi, S. C. Upadhyay, P. K. Ghosh and B. C. Ranu
Green Chem., 2008, 10, 232-237.
12. Prospect of Jatropha Methyl Ester (Biodiesel) in India
Arup Ghosh, D. R. Chaudhary, M. P. Reddy, S. N. Rao, J. Chikara, J. B. Pandya, J. S. Patolia, M. R. Gandhi, S. Adimurthy, N. Vaghela, S. Mishra, M. R. Rathod, A. R Prakash, B. D. Shethia, S. C. Upadhyay, V. Balakrishna, Ch. Ravi Prakash and P. K. Ghosh
International journal of Environmental studies 2007, 64, 659-674.
11. I-/IO3-Assemblies as Promoters of Iodohydrin Formation
S. Adimurthy, G. Ramachandraiah, P. K. Ghosh
Synthetic Commun.2007, 37, 1579-1585.
10. N-Bromosuccinimide a Facile Reagent for the Oxidation of Benzylic Alcohols to Aldehydes
S. Adimurthy and P. U. Patoliya
Synthetic Commun. 2007, 37, 1571-1577.
9. Eco-friendly and versatile brominating reagent prepared from liquid bromine precursor
S. Adimurthy, G. Ramachandraiah, A. V. Bedekar, S. Ghosh, B. C. Ranu and P. K. Ghosh,
Green Chem. 2006, 8, 916-922. (IF 4.192)
8.Description of the Small Plastics Fragments in Marine Sediments along the Alang-Sosiya Ship-Breaking Yard, India
M. S. Reddy, S. Basha S. Adimurthy and G. Ramachandraiah,
Estuarine, Coastal and Shelf Science,2006,, 68, 656-660.
7.Spectrophotometric estimations of Bromide in excess chloride medium,
S. Adimurthy, V.R.K. Susarla, M.P. Reddy and G. Ramachandraiah.
Talanta, 2005, 67, 891-896.
6. Laboratory Studies of Electrochemical Treatment of Industrial Azo dye Effluent
S. S. Vaghela, A. D. Jethva, Bhavesh B. Mehta, Sunil P. Dave, S. Adimurthy and G. Ramachandraiah
Environ. Sci. Tech. 2005, 39, 2848-2855.
5. Reductive dehalogenation of halophenols in sulfite-bisulfate medium.
S. Adimurthy and G. Ramachandraiah
Tetrahedron Lett. 2004, 45, 5251-5252.
4. Electrocatalytic treatment of wastes: Studies on discoloration of an Industrial azo dye effluent
S. S. Vaghela, A. D. Jethva, M. S. Gohil, S. Adimurthy, P. M. Gour, V. R. K. S. Susarla, G. Ramachandraiah, P. K. Ghosh
Annali di Chimica, 2003, 93, (9-10) pp 841-848.
3. Investigations in Nitric acid mediated Dehalonitration of halophenols.
S.Adimurthy, S. S. Vaghela, P. V. Vyas, A. K. Bhatt, G. Ramachandraiah and A. V. Bedekar.
Tetrahedron Lett. 2003, 44, 6393-6395.
2. A Novel Application of Hb-Zeolite in Catalizing Dehalogenation of Halophenols.
S.Adimurthy, G Ramachandraiah, and A.V. Bedekar.
Tetrahedron Lett. 2003, 44, 6391-6392.
1. A new, environment friendly protocol for iodination of electron rich organic compounds.
S. Adimurthy, G. Ramachandraiah, P. K. Ghosh, A. V.Bedekar.
Tetrahedron Lett. 2003, 44,5099-5100.